Introduction
Polycystic Ovarian Syndrome (PCOS) is the most common endocrine abnormality in reproductive aged women affecting approximately 5-10% of this population. (NEJM 1995;333:853) The classic triad of this syndrome consists of chronic anovulation, hirsutism, and obesity. PCOS was first discovered by Stein and Leventhal (Am J Obstet Gynecol 1935;29:181) and its management has confused clinicians ever since. The exciting news recently involves understanding the contribution of insulin resistance to the etiology and treatment of PCOS. This newsletter will review the endocrinopathy and medical consequences of PCOS as well as examine the current understanding of insulin resistance and the use of insulin sensitizing agents.
PCOS
PCOS involves a ‘vicious cycle’ of hormonal imbalance that may begin with a hypersensitivity of the pituitary to GnRH. The pituitary responds with an increase in LH secretion resulting in increased ovarian androgen production by the ovarian thecal cells. This tonic elevation in LH has also been implicated in the well documented, but not well understood increased risk of miscarriage in PCOS patients. The ovarian ‘androgen excess’ of androstenedione and testosterone has several effects: a) inhibiting follicle development and estradiol production; b) increasing the production of dihydrotestosterone (DHT) thereby stimulating excess terminal hair production (hirsutism) and c) increasing peripheral conversion of estradiol to estrone by aromatase.
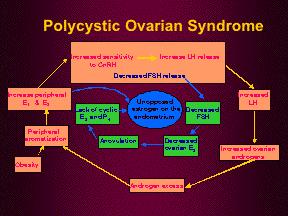
Estrone, although a weaker estrogen, in large amounts has the ability to act on target tissues with similar efficacy as estradiol. Consequently, FSH production is inhibited thereby further preventing follicle development and ovulation. Additionally, estrone proliferates the endometrium unopposed and increases the risk of endometrial hyperplasia and cancer.
To summarize, PCOS is perpetuated by tonic elevations of LH resulting in hyperandrogenemia and chronic anovulation.
Diagnostic Criteria:
PCOS can be diagnosed clinically in women who present with oligomenorrhea (menstrual intervals > 35 days), hyperandrogenism, obesity, and the classic ovarian morphology on ultrasound (after ruling out other endocrine disorders). However, most women with PCOS do not exhibit all of these features and there is a considerable controversy about the definition and required criteria for the diagnosis. A consensus conference of the National Institute of Health on PCOS in April 1990 concluded the following:
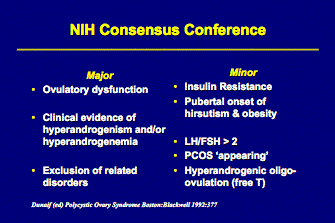
Thus, the minimal criteria include chronic anovulation and hyperandrogenism, and the diagnosis does not require pelvic ultrasound to evaluate morphology for PCOS ‘appearing’ ovaries.
Clinical Presentation and Medical Consequences:
a) Infertility:
Approximately 40% of female infertility factors result from ovulation dysfunction. Women with PCOS may experience a wide range of ovulation dysfunction, from oligo-ovulation to anovulation. In addition to anovulation, other factors appear to be involved, as these women may have a lower rate of conception in response to ovulation inducing agents (clomiphene citrate, gonadotropins) in comparison to women with hypothalamic amenorrhea. Many studies have also described the almost two fold increased miscarriage rate in PCOS, the mechanism of which is poorly understood.
b) Abnormal Uterine Bleeding and Endometrial Hyperplasia
Due to chronic anovulation, women with PCOS usually have irregular menses. The high androgens levels can be converted peripherally to estrogens, particularly estrone, even in the absence of normal ovarian function. As a result, these women are exposed to continuous unopposed estrogen stimulation of the endometrium. Due to anovulation they are deficient in progesterone secretion, which is needed for endometrial differentiation and withdrawal bleeding. Thus, they are at risk for dysfunctional uterine bleeding and, ultimately, endometrial hyperplasia and/or carcinoma.
c) Hyperandrogenism
The clinical features of androgen excess in women with PCOS include hirsutism, acne, male pattern balding (alopecia), and rarely signs of virilization including deepening of the voice, increased muscle mass, and clitoromegaly. Hirsutism occurs in approximately 70 – 80% of PCOS patients (Endocronol & Metab Clinics, 1999;28:398) and is defined as the conversion of vellus (soft, unpigmented) to terminal (thick, pigmented) in a male pattern distribution along sex dependent regions, e.g. upper lip, peri-areola, and lower abdomen. Since all hair follicles are endowed in-utero, there is substantial ethnic variability; up to 33% of non-Scandinavian and non-Asian women have some evidence of hirsutism (Obstet Gynecol Surv, 1999;54:405); also, Asian women may have significant hyperandrogenism without impressive skin manifestations. Virilization is rare and women who present with rapidly progressive masculinizing signs should be evaluated for androgen tumors of the adrenal gland or ovary.
d) Obesity
Obesity is a common, but not necessary, finding in at least 50% to 65% of women with PCOS. Patients usually have central (android) body fat distribution. Android obesity, which is characterized by increased waist-to-hip ratio (>0.80), is correlated with increased plasma testosterone, decreased sex-hormone-binding globulin, hyperinsulinemia, impaired glucose tolerance, and dyslipidemias. (Obstet Gynecol Surv 1999;54:406, PCOS; Cambridge, MA: Blackwell Scientific , 1992 pp359-374)
e) Insulin Resistance and Diabetes
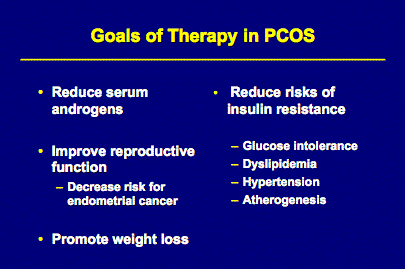
Many women with PCOS exhibit insulin resistance and hyperinsulinemia. Although it is more commonly associated with obesity, it is also found in normal-weight women with PCOS (J. Clin Endocrinol & Metab 1996;81 pp.2854-2964). Because of insulin resistance, women with PCOS are at increased risk for impaired glucose tolerance and diabetes mellitus. A recent study determined that up to 40% of obese reproductive-age women with PCOS had impaired glucose tolerance and 7.5% had diabetes mellitus. In addition, 15% of normal-weight women with PCOS had impaired glucose tolerance and 1.5% had diabetes, a rate almost three-times that of the general population.
The pathogenesis of insulin resistance remains unclear. It has been reported that insulin resistance may be related to decreased insulin receptor autophosphorylation in about 50% of women with PCOS (Endocrinol & Metab Clin; 28(2), 6/99 p. 350). If untreated, insulin resistance leads to diabetes in approximately one-third of patients.
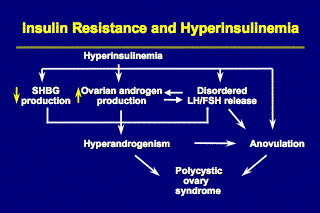
f) Possible predisposition to coronary heart disease:
The presence of obesity, insulin resistance, and lipid abnormalities may predispose women with PCOS to coronary heart disease.
Evaluation:
Polycystic ovary syndrome is primarily a clinical diagnosis, and laboratory findings should only be used to support the clinical findings and rule out other serious disorders. Evaluation should include measurement of thyroid-stimulating hormone (TSH), prolactin, and in some cases, morning 17a- hydroxyprogesterone to rule out late-onset adrenal hyperplasia. Patients, regardless of age, with long-standing unopposed estrogen stimulation, should undergo an endometrial biopsy due to the risk of hyperplasia.
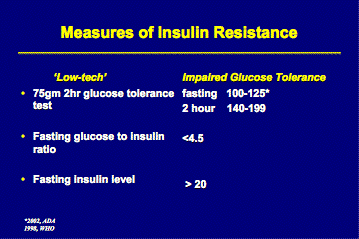
They should also be assessed for insulin resistance and lipid profile abnormalities. Insulin resistance can be evaluated by measuring fasting plasma insulin levels or fasting morning plasma glucose-to-insulin ratio (< 4.5 is highly sensitive and specific for insulin resistance) (J. Clin Endocrinol Metab 1998; 83 pp. 2694-2698), as well as fasting glucose level or 2-hour glucose tolerance test (GTT) – see table. (Endocrinology and Metabolism clinics of North America, June 1999, 397-407).
The diagnosis of PCOS does not require the presence of polycystic ovaries on ultrasound since approximately 20 of fertile women may have polycystic appearing ovaries (Lancet, 1988; 1, pp. 870-872). Polycystic ovaries are present in more than 90% of women with PCOS but may sometimes be absent in women with all the other clinical characteristics of PCOS.
Management:
PCOS is a chronic condition that can be successfully managed with close surveillance. Approaches are directed at preventing potential long-term consequences of chronic anovulation (abnormal uterine bleeding and endometrial hyperplasia), the metabolic abnormalities associated with the syndrome (insulin resistance), treating infertility, as well as improving the external manifestations of hyperandrogenism (hirsutism and acne).
a) Lifestyle Changes:
Weight reduction, diet and exercise are recommended for all women with PCOS.
Weight loss typically results in increased insulin sensitivity, reduced hyperandrogenism, fewer lipid abnormalities and better cardiovascular health. Some studies have also shown a 5-10% loss in body weight may result in a return of ovulatory cycles and a higher spontaneous pregnancy rate. (J. Clin Endocrinology & Metabolism 1999; Vol 84, 1470).
b) Pharmacologic Treatment:
The choice of therapy depends on whether or not the woman wishes to become pregnant.
For women who do not wish to conceive, monthly progestin therapy can be used to prevent abnormal endometrial proliferation by inducing withdrawal bleeding.
Another option for these women is to use low dose oral contraceptive pills (OCP) to regulate the menstrual cycle and provide contraception. The estrogen component of OCPs increases levels of sex hormone-binding globulin to lower free testosterone values and improve hirsutism and acne whereas the progestin component prevents endometrial hyperplasia and cancer.
For women who desire pregnancy, ovulation-inducing agents are utilized and clomiphene citrate is the first line of therapy. Ovulation may be assessed with measurements of urinary LH or with a mid-luteal serum progesterone value. The dose of clomiphene can be increased, until ovulation occurs, up to 250 mg/day and then maintained for up to six cycles. Approximately 80% of women with PCOS ovulate in response to clomiphene, but only about 50% of them become pregnant. (Fertility and Sterility 1984; 42:499). Women who do not respond to clomiphene can be treated with pulsatile administration of gonadotropin-releasing hormone (GnRH) with rates of ovulation and pregnancy of 70% and 45%, respectively (J. Clinical Endocrinol & Metab 1996; 81: 3821).
Antiandrogens may be combined with oral contraceptive pills for the treatment of hirsutism and acne. The most commonly used agent is spironolactone because of its safety and low cost. Several months of treatment may be needed before an improvement in hirsutism is seen.
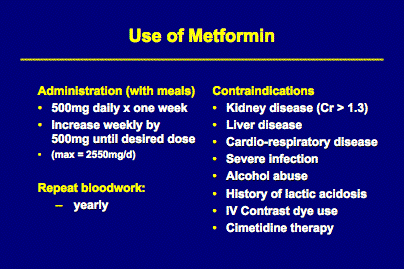
Current Insulin sensitizing agents include the biguanides and thiazolidenediones. Metformin (GlucophageR), a second generation biguanide, works by activating glucose transporters which allow passage of glucose into hepatic and muscle cells thereby decreasing peripheral insulin resistance. Metformin does not stimulate insulin release and, when given alone, does not cause hypoglycemia (American Society for Reproductive Medicine, a practice committee report, April 2000). In a randomized trial, obese women with PCOS were given 500 mg of metformin three times daily for 35 days or placebo. 34% of the women in the metformin group ovulated spontaneously during treatment with metformin alone, as compared with only 4% in the placebo group. The subjects who remained anovulatory after 35 days, continued into the second phase of the trial, in which clomiphene was added to both groups. 90% of women who received combined metformin and clomiphene ovulated compared to only 8% in the group given placebo and clomiphene. (NEJM, 6/25/98, pp.1876-1880).
The Thiazolidenediones are under active investigation for treatment of PCOS. They act by binding to peroxisome proliferation activator receptor gamma, which decreases peripheral insulin resistance. The available drugs in this class include rosiglitazone (AvandiaR) and pioglitazone (ActosR). Troglitazone (RezulinR) was removed from the market due to several reports of hepatic failure.
©Mark P. Trolice Copyright 2006

